
Strategies to help reduce or avoid Severe Suffering
There are a number of practical elements that can contribute towards reducing or avoiding severe suffering, including:
We have also developed a 'Road Map towards Ending Severe Suffering' to bring together these elements into a practical strategy.
Refinement
Refinement can be defined as any measure that will reduce suffering or improve welfare, whether this is applied to scientific procedures, housing, transport, husbandry procedures (including marking for identification and cage cleaning), the animals’ environment (e.g. temperature and light levels), welfare assessment, humane endpoints or humane killing techniques.
This is clearly critical to tackling severe suffering, but it can be easy to become complacent about implementing refinement. Some key points to remember are: refinement is not a ‘one-off’ event at the project planning stage, and it is not just a matter of following in-house refinement protocols. There may be new information and approaches outside your establishment, and you may need to search for these. Open minds are essential - if there are any questions about the effects on animal welfare or the science, the course of action should be to evaluate these and carefully consider the results. Lastly, communicating about refinement should be a two-way street, and it is especially important for scientists to share good practice with their peers.
- Search the literature for guidance on refining the specific procedure in question. An internet search on [animal model], [species] and [welfare] or [refinement] can be a useful starting point as well as dedicated discussion fora such as COMPMED, LAREF and VOLE (these all require membership). The person responsible for ensuring that staff have access to species-relevant information within each establishment should be able to provide additional information (this is the Named Information Officer in the UK). Expert working group reports are available for some severe procedures; for example, RSPCA-convened expert working groups have produced reports on refining procedures involving seizures, convulsions and epilepsy; experimental allergic encephalomyelitis; sepsis; and rheumatoid arthritis.
- Consult researchers, animal technologists and veterinarians with experience in the field, and relevant societies, organisations and user groups.
- Raise the issue within your establishment – ask the AWERB, AWB or ACUC for a discussion or to provide advice, consult the veterinarian or the person(s) responsible for disseminating information about the 3Rs.
- Ask the regulator for advice. For example, the UK Home Office Inspectors within the Animals in Science Regulation Unit often provide advice on refinement and encourage establishments to share good practice.
- Go to meetings that address refinement, for example those convened by the RSPCA, IAT, LASA, LAVA and the NC3Rs (or equivalent bodies in your country). Get refinement added to the agenda at meetings held by your own scientific or professional bodies, for example as a presentation, symposium or poster.
- Include information on refinement in posters, presentations and publications. This is relevant to the science and editors or peer reviewers should not insist that you remove it. If you have any issues, use the ARRIVE guidelines to help make your case (especially if the journal has endorsed these).
- RSPCA user-friendly check sheets on housing, husbandry and care of a range of commonly-used species, plus refining cage change and humane killing, can be downloaded on our Housing, husbandry and care page
- BVA(AWF)/FRAME/RSPCA/UFAW Joint Working Group on Refinement (JWGR) reports address refinements in both procedures and housing and care
- RSPCA/UFAW Rodent Welfare Group meetings
- UFAW/RSPCA report on Refining Rabbit Care
- RSPCA resources on housing, husbandry and care of Zebra danio and Xenopus spp
- RSPCA/APHA meetings on the Welfare of Farm Animals in Research
- UK NC3Rs Resource Hub
- Norecopa 3R Guide
- Animal Welfare Institute Enrichment and Refinement Databases
Welfare Assessment
The term 'welfare assessment' refers to monitoring animals for signs of pain, suffering and distress associated with procedures or their effects, as well as to the day-to-day assessment of all animals to detect health or welfare issues so they can be dealt with. It is also good practice to define and include indicators of positive wellbeing, such as appropriate levels of grooming and social interaction, when devising protocols for monitoring animals.
Effective welfare assessment is especially important with respect to severe suffering, as it can help to prevent pain and/or distress reaching ‘severe’ levels and better inform refinement approaches and the implementation of humane endpoints.
In the UK, project licence holders and personal licensees have a legal responsibility to ensure that animal suffering is minimised during all regulated procedures. This includes establishing a specific welfare assessment protocol tailored to the species and procedure, at the project planning stage, as well as being ultimately responsible for the monitoring and care of animals used in procedures. It is critical that there is a good understanding of the normal physiology and behaviour of the species (and strain, if appropriate) and of how deviations from normality can affect welfare and provide an indicator that there could be a problem.
You may be able to use some of the parameters you are monitoring for scientific purposes as part of the welfare assessment, e.g. if animals have been implanted with heart rate or blood pressure telemetry devices as part of the procedure. Whichever behavioural or physiological indicators you choose to assess welfare and implement humane endpoints, it is good practice to consider them with care and think about how they will be used in practice, rather than cutting and pasting from other licences or standard protocols. Animal technologists and veterinarians can provide expert advice to help define welfare assessment protocols and to assist in their application, and you can ask your AWERB, AWB or ACUC for discussion and guidance. It is helpful to include the final welfare assessment protocol in publications, e.g. as supplementary online material.
Animal technologists and vets play an essential role in the day to day care and welfare monitoring of animals. Named persons also have legal responsibilities with respect to ensuring that animals are effectively monitored, and suffering dealt with. Good communications and relationships with researchers, and support from management and the AWERB, will help to ensure that animal technologists and vets have constructive input into welfare assessment protocols - and they should also be involved in ‘actual severity’ assessments and wider retrospective reviews of severity.
The AWERB should insist that all project applications are accompanied by a welfare assessment protocol that has been tailored for the species and strain. This should demonstrate understanding of the welfare impact of the animals’ lifetime experiences, including the procedure and its effects. The protocols do not have to be included in the licence application, but it is important for the AWERB to see these and understand how they will be used day to day. Ideally, the researcher would be able to discuss how welfare will be assessed, and humane endpoints implemented, with the AWERB in person.
If an applicant cannot adequately set out the welfare issues, or if the AWERB has concerns about the potential for severe suffering, a welfare pilot study may be requested (see section 2.3.4 of the JWGR report (PDF 818KB)). This is a useful way of establishing whether the proposed protocol will cause severe suffering, and defining welfare assessment and potential refinements.
Two useful guidance documents for AWERB and AWB members are the RSPCA Lay Members’ Handbook (PDF 6,742KB) and the RSPCA/LASA Guiding Principles on good practice for AWERBs (PDF 1.41MB). Both give useful advice on how AWERB or AWB members can promote the implementation and dissemination of refinements. Although the Guiding Principles are written for UK AWERBs, many of them are also useful for AWBs and ethics or animals care and use committees operating worldwide. The Lay Members’ Handbook is specifically intended for an international audience.
Guidance on identifying suitable behavioural and physiological indicators of discomfort, pain or distress, defining appropriate recording systems and setting out effective monitoring protocols can be found in:
- JWGR (Joint Working Group on Refinement; 2011) A guide to defining and implementing protocols for the welfare assessment of laboratory animals (PDF 818KB). Laboratory Animals 45: 1-13
- European Commission (2012) Working document on a severity assessment framewor (PDF 720KB)
- European Commission (2013) Examples to illustrate the process of severity classification, day-to-day assessment and actual severity assessmen (PDF 840KB)
- ILAR (2008) Recognition and Alleviation of Distress in Laboratory Animals. Washington, DC: National Academies Press
- ILAR (2009) Recognition and Alleviation of Pain in Laboratory Animals. Washington, DC: National Academies Press
- NC3Rs welfare assessment resource //www.nc3rs.org.uk/welfare-assessment
- National Health and Medical Research Council (2008) Guidelines to Promote the Wellbeing of Animals Used for Scientific Purposes: The Assessment and Alleviation of Pain and Distress in Research Animal (PDF 1.32MB). Canberra: Australian Government
- Wells DJ et al. (2006) Assessing the Welfare of Genetically Altered Mice (PDF 48KB)
- Assessing the Health and Welfare of Laboratory Animals (AHWLA) www.ahwla.org.uk/index.html
Humane Endpoints
A humane endpoint is, effectively, a limit to the level of suffering an animal can experience within a given scientific procedure. It is a defined point at which an experimental animal’s pain and/or distress is either ended or reduced, within the context of the scientific endpoints to be met. Actions to end or reduce suffering include humane killing, removal from the study, provision of analgesia, or any other appropriate measure to reduce suffering such as heat pads or dietary supplements. Death or severe morbidity as an endpoint should always be challenged in any field of research.
Under UK legislation, the responsibility for the implementation of humane endpoints sits with the personal licensee. However, discussion with ‘named persons’ and the AWERB will help to identify appropriate criteria for humane endpoints, and when to implement these, which can be included in the project licence application.
Experimental protocols should be designed to allow for the earliest possible alleviation of suffering. This may require a shift towards ‘mechanism driven’ (biomarker or pathway specific) experimental endpoints in some disciplines rather than phenotypic or disease driven endpoints. It may be argued that more useful data can be obtained from an animal not experiencing severe morbidity or imminent death, even if they are being used to model (or test potential therapies for) severe clinical disease. For ongoing studies, it is important from time to time to reflect on current endpoints and to consider whether further refinements could be applied.
The AWB/AWERB should ask for explanations of humane endpoints, including how they are defined, refined and implemented. They can also ask to see, and discuss, animal ‘fate’ data, including a breakdown of animals humanely killed as part of the experiment, found dead, killed because they are close to a humane endpoint, or because they are not needed (surplus). This will allow the establishment to monitor wastage, identify where endpoints may need to be revised and see where additional welfare monitoring should be applied.
For further information, see www.humane-endpoints.info and www.nc3rs.org.uk/humane-endpoints
Road Map
The ‘Road map’ is a series of practical steps that will enable establishments to identify ways to reduce, avoid and ultimately end severe suffering. This approach is linked to AWERB tasks and was developed by the RSPCA following consultation with a number of research establishments. A key principle of the Road map is an ‘audit’ of procedures to establish how well current refinement practices are working and to identify any areas where further refinement can be applied. In order for this to be achieved it is essential that researchers work with their ‘named persons’ and develop a team approach to the alleviation of suffering.
A paper outlining the concept was published in 2014 Road map paper (PDF 80KB)
The key stages to the road map are shown here:
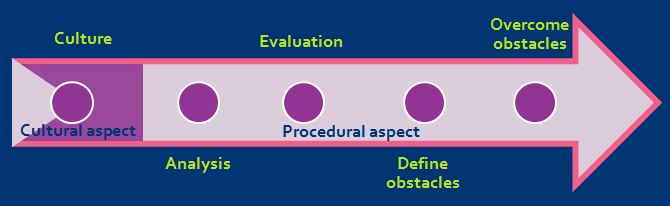
and are expanded upon below:
A fundamental requirement for ending severe suffering is the collective agreement, within the research establishment, that this is both desirable and possible – and worthy of the necessary time and resources. A progressive, open minded and caring research culture should be willing to embrace these concepts.
For this approach to work, it is essential that there is a demonstrable ‘culture of care’ within the establishment, including a shared responsibility and accountability for animal welfare, robust support for named persons, and a positive collaborative relationship between animal technologists, veterinarians and researchers.
In the UK, named persons and other staff should be supported by the AWERB on welfare and ethical issues, which should help individuals to contribute towards and maintain a good culture of care.
The first of the procedural steps is to establish the extent of the issue in-house, beginning with a ‘severity audit’ of all protocols, procedures and models to identify any that have the potential to cause severe suffering. These procedures can then be reviewed, using records of day-to-day observations of the animals, to identify those where the animals experience severe suffering (for example where the actual severity, required for reporting purposes under EU Directive 2010/63, is severe).
Step two is to take the audit information and evaluate the following:
- why each procedure was used and which factors resulted in it being potentially or actually severe;
- whether that level of suffering was really necessary to achieve the scientific objective;
- what proportion of animals suffered severely;
- what refinements were already in place and whether there is potential for further implementation of all Three Rs.
A critical review of the justification for, and necessity of, severe procedures should reveal any scientific obstacles that need to be overcome to end severe suffering, taking an honest and realistic view of the impact (either negative or positive) on the scientific objectives of avoiding or refining the severe procedures.
Any obstacles to ending severe suffering should be clearly set out and the genuine impact evaluated.
Critical evaluation of obstacles identified in the previous step may suggest opportunities to overcome them. It may be that a few changes to current protocols are all that are needed to avoid severe suffering. It some cases, avoiding or ameliorating severe suffering may be more challenging. In all cases, some progress should be possible even if this requires a long-term plan and further research or protocol development.
There should be a coordinated effort from researchers both to develop and validate humane endpoints in order to limit suffering experienced by animals and to develop and adopt alternative, non-severe, approaches.
It may be useful to apply a ‘stretch objective’ to ending severe suffering by imposing a challenging but achievable time point after which no further severe studies would be undertaken. This target may be different depending on the model or procedure being used and the specific obstacles that are identified. However, having a fixed point in time to work towards can be a powerful motivating force for achieving challenging goals.
Additional Road Map resources, including a poster and AWERB materials can be found on the resources page
Case Studies
The following case histories illustrate where refinement has been applied to severe models or procedures and the benefits that have accrued:
Case history 1 (ALS): Changes to welfare assessment and application of refinement help to reduce suffering in a mouse model of amyotrophic lateral sclerosis.
The SOD1G93A mouse is a commonly used model of amyotrophic lateral sclerosis (ALS), a form of motor neuron disease which is rapidly progressive and fatal in humans. Current treatments only have a limited impact, so the SOD1G93A mouse is used to test potential therapies. Testing treatments that are intended to slow disease progression can require animals to experience clinical signs that can cause severe suffering.
The life experiences of SOD1G93A mice were reviewed and refined, and a new Standard Operating Procedure set out for caring for the animals, including:
- early screening of motor function and muscle characteristics, to select which new drug candidates to take forward without mice suffering extreme motor deficits;
- righting reflex checked twice daily from 100 days (or earlier if motor problems are apparent);
- non-particulate litter and nesting material, as animals may have difficulty grooming;
- long sipper tubes and mash provided in a dish on the cage floor, to cater for disabled animals;
- any dehydration treated with an i.p. injection of sterile saline, followed by close monitoring;
- husbandry refinements, including carrying nesting material (not litter) over from soiled to clean cage to reduce aggression, changing gloves and cleaning work surfaces between handling male and female mice. Fun tunnels are provided but removed at 100 days to prevent disabled mice from becoming trapped.
All investigators and animal technologists have a copy of the standard operating procedure (SOP), which has helped to avoid and reduce severe suffering.
Source: Hawkins et al. (2012) (PDF 260KB) Animal Technology and Welfare 12: 49-58
Case history 2 (cuprizone): Use of imaging techniques and additional refinements reduce severity from severe to mild in a model of demyelination.
Animals are used to study demyelinating diseases of the nervous system, in which the myelin sheath that insulates neurons (nerve cells) is damaged. These include multiple sclerosis, Charcot-Marie-Tooth disease and central nervous system neuropathies. In a commonly used model, mice are fed cuprizone, which causes neurons to degenerate throughout the nervous system including the brain. Potential drugs and therapies can then be evaluated for the disease under study.
Six weeks of 0.2 per cent cuprizone in the diet causes a significant level of reversible demyelination in mice, which becomes irreversible if exposure to cuprizone is continuous for up to 12 weeks. Mice treated with cuprizone invariably exhibit spontaneous seizures (fits) and mortality can be up to 50 per cent in males. Treatment with cuprizone can therefore be severe.
A pharmaceutical establishment wanted to refine the use of cuprizone and, after evaluating the literature, they set up this protocol:
- Female mice weighing at least 25g were used – according to the literature, female mice are less susceptible to cuprizone
- 0.2 per cent cuprizone was incorporated into chow pellets, which mice prefer to powdered diet and find easier to handle
- Animals were housed in a ‘quiet’ area of the animal facilities with low traffic
- The number of interventions was reduced by combining various activities within a single handling session, e.g. by weighing and checking the animals during cage clean
- MRI techniques were used to assess demyelination more accurately and refine humane endpoints. Scanning sessions lasted 15 minutes, under isoflurane anaesthesia
As a result, the actual severity was mild for all animals and the animal model was robust. MRI was also used to implement reduction, as longitudinal data was obtained from a single animal.
Case history 3 (fungal): Development of an early humane endpoint in a model of fungal infection.
Fungal LD90 tests are conducted to characterise the infectivity of a fungus (e.g. Aspergillus) or to evaluate the effectiveness of antifungal treatments. For example, the antifungal treatment may be started 24 hours after infection and continued for seven days, with mortality then recorded over the following week.
In a study to evaluate the potential to use body temperature as a predictor of death in fungal (Candida) LD90 tests, male CD1 mice (4 to 5 weeks old and between 18 and 20 g) were immunosuppressed with cyclophosphamide and infected with either C. albicans, C. guillermondii or C. glabrata. Body temperature was measured using an implantable transponder and a high performance non-contact thermometer. The non-contact thermometer gave reliable results and enabled an endpoint of 33.3 oC to be defined, which would have been able to predict mortality in 67 per cent of the mice.
Source: Warn et al. (2003) Laboratory Animals 37: 126-131
This builds on earlier work on humane endpoints in rodent infection studies see: Acred et al. (1994) Laboratory Animals 28, 13-18
Case history 4 (RA): Adoption of a range of refinements combine to reduce suffering in a mouse model of rheumatoid arthritis.
A pharmaceutical company introduced the G6PI, CIA and CAIA mouse models of rheumatoid arthritis, which have the potential to cause severe suffering. This prompted a re-evaluation of the company’s welfare scoring sheets and husbandry refinement protocols, with the aim of reducing suffering. The researchers, animal technologists and Named Animal Care and Welfare Officer worked together to tailor and refine monitoring systems, husbandry and procedures.
Mice used in G6PI and CAIA studies were very carefully monitored by researchers and technologists, to identify indicators of adverse effects and collate data on weight loss and disease scores. The observations were specific to each model, although standardised terminology was created to describe indicators. As a result, the following refinements were adopted:
- the humane endpoint for weight loss was reduced from 25 per cent to 20 per cent, and another endpoint added of a 15 per cent weight loss that persisted for 5 days;
- the tailored indicators (such as soft stools for CAIA) enabled study length to be reduced; e.g. the CIA studies were reduced from 30 days to 20;
- disease scores were revised to include a range of indicators, as opposed to paw volume only, capturing severity more effectively and enabling endpoints to be further refined;
- additional refuges are provided for DBA/1 male mice, eliminating aggression;
- non-tangling nesting material is provided;
- when mobility is restricted, longer sipper nozzles are fitted and food given in dishes on the cage floor;
- the Mouse Grimace Scale is used to help assess acute pain.
If you have a case study that you would be prepared to share on this site, we would be pleased to hear from you: research.animals@rspca.org.uk