
What can cause Severe Suffering.
Severe suffering can occur for three main reasons:
- Some procedures or ‘models’ are inherently more likely to cause severe suffering
- In some cases, a combination of less severe factors can lead to an increase in overall suffering; this is often termed 'cumulative severity'
- Mortality may involve severe suffering; this includes both unexpected mortality and ‘death as an endpoint’
Severe models and procedures
Some procedures are more likely to be severe than others. Examples include control groups in some vaccine studies, or some ‘models’ of diseases or conditions that cause high levels of suffering in clinical cases. Some examples of potentially severe procedures are listed on pages 8 to 9 of the European Commission report (PDF 192KB) on prospective severity classification. Further examples are listed below, on the basis of the scientific literature and discussions with researchers, animal technologists and veterinarians working in academia and industry in the UK. These are listed as guidance only; their actual severity will depend upon individual experimental protocols and the efforts that have been made to refine these.
- Severe cancer models - involving large tumours, resection, bone metastasis, brain tumours, pancreatic tumours
- Irradiation with reconstitution of bone marrow
- Some heart disease models: myocardial infarction induction; Monocrotaline-induced PAH; transverse aortic constriction/banding
- Spinal cord injury models
- Infectious disease models
- Tamoxifen as inducers of gene function
- Vaccine testing for regulatory purposes
- Toxicology / ecotoxicology protocols involving many species
- Models of Motor Neuron Disease
- Cerebral malaria in rodents
- Multi-organ failure models
- Pancreatitis models
- Demyelination of CNS
Cumulative severity
Apart from experimental procedures, each animal used in research and testing experiences many other events during their lifetime, some of which may be painful, distressing, or may influence the animal’s ability to cope with regulated procedures. These include transport, marking for identification, capture, handling, restraint, laboratory housing and husbandry, scientific procedures and the after effects of these, and humane killing.
Multiple events and stressors like those listed above can affect overall severity, and it is important to consider how their effects may interact with one another. The term ‘cumulative severity’ is often used, but harms do not ‘accumulate’, or simply add up - although animals may become sensitised to certain procedures (e.g. repeated injections for a mouse), so the suffering associated with each one is increased. As another example, if recovery time is not sufficient following stressful events, such as cage change, before conducting a procedure, then the severity of the procedure may be elevated. The cumulative impact of some procedures (e.g. surgery without the most effective perioperative analgesia regime) may be long-lasting or permanent.
Alternatively, animals may habituate (become used) to repeated procedures, which can reduce suffering, especially if they can be trained using positive reinforcement techniques to avoid restraint.
It is critically important not to make subjective assumptions about cumulative severity either increasing or decreasing - expert input and monitoring systems are both necessary to ensure that welfare issues, and refinements, are detected.
These are closely linked and can be used to continually inform and update one another, as shown here.
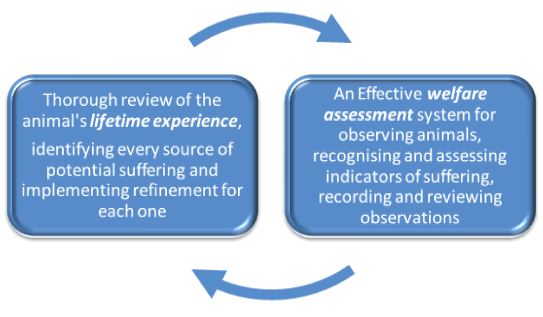
The best time to review and consider the lifetime experiences of each animal is at the project planning stage, with input from people with different expertise including the researcher, the veterinarian and animal technologists. The AWERB, AWB or ACUC should be able to provide useful input. A major aim is to identify as many sources of suffering (harms) as possible, so that appropriate refinements can be researched and included in experimental protocols and within housing, husbandry and care, wherever this is compatible with the science. Another important aim when reviewing the animal’s lifetime experience is to identify and define humane endpoints.
Considering lifetime experiences at the project planning stage can help to determine indicators of suffering that are tailored to the species, strain (if appropriate) and procedure and can be used to assess welfare day to day throughout the procedure. If there are good welfare assessment systems in place for recognising and assessing behavioural and physiological indicators of pain or distress, suffering can be acted upon sooner and more effectively - which will reduce severity. The ‘six level category’ approach in Appendix 1 of the EC Severity Assessment Working Document (717KB) is helpful when defining an assessment system. Standardised terminology with respect to welfare indicators can also be useful in order to ensure that all staff are ‘on the same page’ (for example: www.mousewelfareterms.org) and to avoid confusion that could delay intervention.
During and after procedures, records of ‘cageside’ observations can be reviewed to assess how accurately harms were predicted, how severely animals were affected and how effective any refinements have been. All of this information, obtained using robust welfare assessment protocols, should then complete the circle and help to implement refinement in future projects. The regulator within the European Union also requires that ‘actual severity’ is reported, but this is just a snapshot of the animal’s ‘worst day’ and should only form part of the overall assessment of the animal’s experience.
Mortality
From animal welfare, ethical and usually scientific aspects, mortality should be avoided. However, in practice, animals can die in the laboratory (as opposed to being humanely killed to implement humane endpoints or at the end of a procedure). For example, many commonly used mouse strains have a background incidence of morbidity and mortality, and in large facilities the sheer number of animals makes it unfortunately inevitable that some will be ‘found dead’. The essential question is how far mortality can be prevented, for example by:
- working to improve predictors of death within procedures so that humane endpoints can be refined,
- defining predictors of background morbidity or mortality (unconnected with procedures), so that these can be better identified within day to day monitoring protocols, or
- challenging regulatory requirements for death or morbidity as an endpoint
Under the EU Directive (PDF 720KB), if animals are ‘found dead’ it is assumed that severity was severe unless there is evidence otherwise. Exceptions can be made in some cases, for example if death is rapid and observed, without signs of suffering, or if prior knowledge of the model or intervention would indicate that suffering was unlikely – but these are likely to be few. The emphasis should be on improving the ability to detect indicators of mortality, and on challenging any assumptions that mortality is ‘inevitable’ or that endpoints cannot be refined. Perceptions about the ability to predict death often change; for example, telemetered body temperature using microchips has greatly improved the ability to predict death in a number of fields. It is good practice for researchers and named persons to keep up with the literature and to identify any new approaches that may be suitable for trialling at the facility.
Directive 2010/63 EU (PDF 720KB) states that ‘Death as the end-point of a procedure shall be avoided as far as possible and replaced by early and humane end-points’. Any perceived or actual regulatory requirements for death as an endpoint should be rigorously examined and critically challenged. For example, the OECD (PDF 160KB) recognises that ‘with increasing knowledge and experience, investigators in animal research will be able to identify more specific, early humane endpoints in the form of clinical signs for impending death or severe pain and distress. This would permit international harmonisation of these humane endpoints.’ Researchers and establishments should challenge regulatory bodies to accept evidence that death can be predicted and to accept data obtained from tests in which humane endpoints have been defined and implemented.